TruCheck Cancer Early Detection
The stakes of preventive medicine are particularly crucial in oncology, where the effectiveness of treatments is multiplied by early management of the disease.
We have certain clinical requirements that you must meet to be eligible to take this test. You will need to complete the online consent form when ordering to confirm these requirements.
To take this test you must:
Be 40 years or older. (People aged 35-40 are accepted subject to being considered high risk, please inquire.)
Do not currently have symptoms of cancer. If you have suspicious symptoms, you should consult your GP urgently.
Do not currently have any clinical or radiological suspicion of cancer.
Have never been diagnosed with any type of cancer.
Have never received treatment for any type of cancer.
Have not received a blood transfusion in the 72 hours preceding your blood collection appointment.
Please ensure you carefully review the eligibility criteria mentioned above before booking the test, as failure to meet the criteria or any subsequent changes may result in cancellation of the test.
The test is reserved for asymptomatic adult without a prior cancer diagnosis and over the age of 35.
Attention ! This test may have repercussions on your psychological state. Indeed, it is possible that the results could generate strong emotions, the risk of discovering information that could cause worry or anxiety.
Moreover :
The information included in the kits is in no way intended to replace the relationship you have with your doctor or other healthcare professional. Before initiating, stopping or changing any treatment or medication, it is imperative to consult your doctor.
This service is of a general nature and is not intended to be a substitute for a medical examination, professional medical advice, medical advice, diagnosis or specific treatment for your health. It is strongly recommended that you always seek qualified and regulated healthcare professionals with any questions you may have regarding a specific medical condition you may be concerned with.
Blood test for early detection of tumor cells in 70 organs of your body.
TRUCHECK HAS BEEN TESTED AND VALIDATED ON MORE THAN 60,000 PEOPLE. FDA & CE CERTIFICATION
Consent form
- Our doctors will check the relevance and feasibility of the test for you.
An analysis kit
- Blood test to be carried out in the laboratory of your choice. 3 tubes of 10ml. 10ml. 5ml.
- Manual and explanatory video
Results within 2/3 weeks.
Positive: a personalized patient journey is planned as well as a 2nd control test which will be offered to you free of charge.
- Negatives: the test is finalized.
£ 1299
The cost of the blood test to be carried out in the laboratory of your choice, you do not need a prescription to take the blood test. If this is requested, please contact us.
What is the TruCheck Cancer Early Detection test?
The TruCheck™ Early Cancer Detection Blood Test is a blood test that revolutionizes the ability to detect cancer in its early stages.
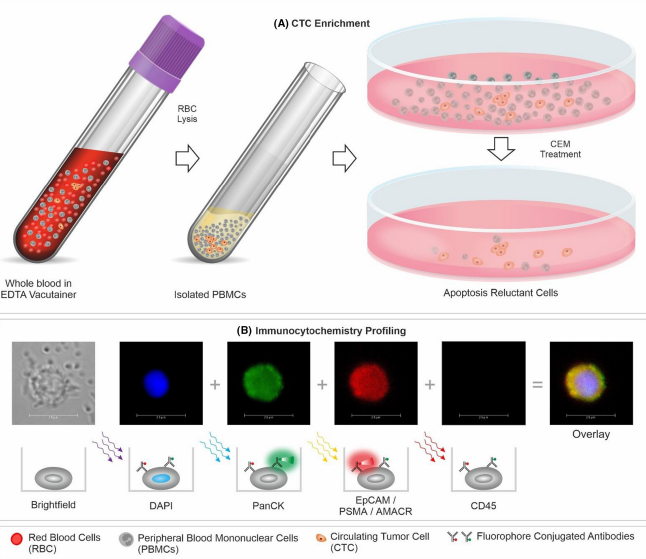
A non-invasive procedure, this test requires a simple blood test appointment. It is the only test available to check for the presence of circulating tumor cells (CTCs), detecting even the smallest existence of cancer from a small blood sample.
Very precise, it not only identifies if you have CTC*, but also where they come from. This means you know where the primary tumor is in your body, speeding up diagnosis and treatment, and allowing doctors to focus treatment on the areas needed.
We offer it as a stand-alone test and recommend that it be added to your annual health check.
It does not replace more specific diagnostic tests for particular cancers, but often identifies them at an earlier stage and allows us to take faster and more effective action.
How is this test revolutionary?
Launched in the UK in late 2022 after rigorous clinical trials, this test is brand new to the diagnostic screening industry. It is the only test available to check for the presence of circulating tumor cells (CTCs), detecting even the smallest existence of cancer from a small blood sample.
This test has received Breakthrough Designation, which is granted by the FDA to innovative therapies. This is a priority status granted by the American Food and Drug Administration to a medical device that is likely to provide a decisive therapeutic advance.
The test meets all marketing authorizations including the CE standard.
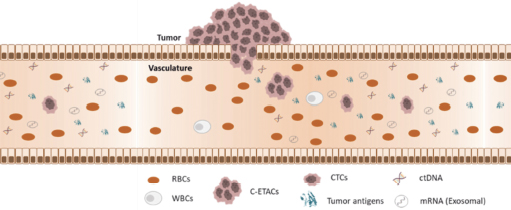
During tumor development, some cancer cells can break off from solid tumors and infiltrate the bloodstream. These are circulating tumor cells (CTC). Tumor cells will also die, disintegrate and release fragments of their genetic material, circulating tumor DNA, into the blood.
(tcDNA).
A simple process
- If you are eligible for the test (be over 35 years old, have not had cancer, etc.) we will send you a test kit and you will need to take a blood sample in a laboratory of your choice.
- The blood tubes will then be sent to our laboratory by DHL with the pre-paid return envelope.
- The results will be returned to us within 2/3 weeks of receipt of your samples. We will organize a call together to comment on the results with you and consider a follow-up protocol if necessary.
- Avoir 40 ans ou plus. (Les personnes âgées de 35 à 40 ans sont acceptées sous réserve d'être considérées à haut risque, veuillez vous renseigner.)
- Ne pas avoir actuellement de symptômes de cancer. Si vous avez des symptômes suspects, vous devez consulter votre médecin généraliste de toute urgence.
- Ne pas avoir actuellement de suspicion clinique ou radiologique de cancer.
- N'avoir jamais été diagnostiqué avec un quelconque type de cancer.
- N'avoir jamais reçu de traitement pour un quelconque type de cancer.
- Ne pas avoir reçu de transfusion sanguine dans les 72 heures précédant votre rendez-vous pour le prélèvement sanguin.
What is included
1
A test kit
A collection kit will be sent to your home and you will send your blood samples by pre-paid DHL envelope to the laboratory. These samples are very simple and are explained by instructions and a video.
BODYCHECKUP is committed to protecting and respecting your privacy. This policy (together with our terms of use and any other documents referred to in it) sets out the basis on which any personal data we collect from you, or that you provide to us, will be processed by us through this site.
2
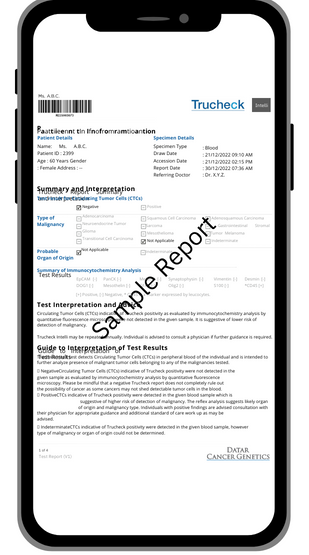
A detailed test report.
After 2/3 weeks, you will receive a complete report on the presence or absence of tumor cells in your blood.
• A positive result means that CTCs have been found in your blood. The test results will indicate the location of the cancer type with high accuracy. This means we can tell you where the cancer started to grow. A specialist doctor will call you personally to discuss your results and what they mean.
A personalized patient journey is planned as well as a 2nd control test which will be offered to you free of charge.
• A negative result means that at the time of the test, there were no detectable cancer cells for the cancers included in the test circulating in your blood. This doesn’t mean you’ll never develop cancer. For all cancers not covered by the test, no conclusions should be drawn from a negative result.

3
In case of positive test
In the event of positive results, the scientific committee will be consulted and a specialized doctor will guide you to the best specialists to take care of you.
Our doctors will help you:
- Management of your medical file by our scientific committee.
- Call or Video consultation with a specialist.
- A personalized patient journey is planned.
- A 2nd control test which will be offered to you free of charge.
OUR ETHICAL CHARTER
CE Certified Lab
We Protect Your Privacy
Questions ? Check out our FAQ
Thucheck from Datar Center Genetics is a blood test that has received the “Breakthrough Device” label (considered revolutionary) by the US Food and Drug Administration (FDA).
This non-invasive test can detect more than 70 different types of solid tumors whether metastatic or not and requires only a 25 ml blood sample.
Trucheck detects circulating tumor cells (CTCs).
CTCs are not detected in individuals without cancer or in those with benign (non-cancerous) diseases. CTCs enable the detection of cancers with high sensitivity and enable differentiation between cancer patients and healthy individuals with high specificity.
The test has a sensitivity of 88.2% in detecting cancers of all stages and types. Additionally, it has an accuracy of 93.1% in determining the tissue or organ of origin in positive cases. This means that even early-stage cancers are reliably detected. Additionally, the specificity of Trucheck is 96.3% in asymptomatic individuals and 95% in individuals with benign tumors.
The test will give a negative or positive result. A negative result suggests that no circulating tumor cells were detected in the donated blood sample. A positive result occurs when CTCs are detected in the donated blood sample, suggesting malignancy with a sensitivity of 99.9%. This can be further analyzed to determine the tissue or organ of origin.
In certain circumstances, an imbalance in the composition of the microbiota can manifest itself, characterized by an excessive or insufficient presence of certain bacteria compared to normal, which is called dysbiosis.
Evidence suggests that this disruption of the balance of the intestinal microbiota could be linked to problems such as depression, obesity, chronic inflammatory diseases, Alzheimer's, Parkinson's or certain cancers.
Maintaining the balance of the intestinal microbiota is therefore of crucial importance for:
Guarantee the proper functioning of its various functions.
Limit its involvement in the development of metabolic, digestive, and other pathologies.
In order to achieve this objective, it is essential to know the composition of one's microbiota to detect possible imbalances and identify the possible presence of pathogens. With this in mind, we offer you the “intestinal microbiota” assessment, which allows you to establish a detailed map of your intestinal microbiota.
Nearly one in two people will be diagnosed with cancer in their lifetime and 50% of these people will be diagnosed after their cancer has spread significantly.
Cancer is the leading cause of premature mortality in France, ahead of cardiovascular diseases. In 2023, it is estimated that more than 433,136 new cases of cancer will be diagnosed in France and that 3.8 million people live in France today with a cancer diagnosis.
The goal of screening is to be able to diagnose cancer at an early stage, even if it does not yet produce symptoms. Very early treatment promotes the chances of recovery and limits the after-effects linked to the disease and certain treatments.
The test is reserved for asymptomatic adult men and women without a prior cancer diagnosis and over the age of 35.
A positive result means that CTCs have been found in your blood. The test results will indicate the location of the selected cancer type with high accuracy, and in other cancers the tissue of origin can be identified with reasonable accuracy. This means we can tell you where the cancer started to grow in your body.
If you test positive, your doctor will personally call you to discuss your results, what they mean, and guide you in your next steps, including a referral. We have established a patient pathway if you get a positive result.
A negative result means that at the time of the test, there were no detectable cancer cells for the cancers included in the test circulating in your blood. This doesn't mean you'll never develop cancer. For all cancers not covered by the test, no conclusions should be drawn from a negative result.
Sensitivity is the ability to correctly identify people with cancer. All TruCheck tests detect early signs of cancer at least 88% of the time. So the test will correctly identify at least 88 out of 100 people with early signs of cancer.
Body CheckUp is a subsidiary of Kensington International Clinic (https://kensingtoninternationalclinic.co.uk/en/)
Located in the Kensington district of London, Kensington International Clinic (La Maison Médicale) is an international clinic created by a group of French doctors in 2010 and which includes a complete health center and 3 centers of excellence: gastrointestinal, gynecology, mental health and well-being. The clinic has more than 40 doctors and has welcomed nearly 20,000 patients. The clinic created the KIC ACADEMY which works in particular in the field of microbiota and preventive detection of cancers. La Maison Médicale has become a pioneer in these exciting areas of medicine, offering very effective diagnostic and treatment programs specifically adapted to each patient.
Specificity is the ability to correctly identify people who do not have cancer.
All TruCheck tests have a specificity of 99%. So the test will correctly identify 99 out of 100 people without cancer. This means you have a low probability of receiving a false positive result and undergoing unnecessary medical procedures.
Certain clinical requirements must be met in order to be eligible for this test.
Before registering, a pre-consultation call with our doctor will be arranged.
To take this test, you must:
Be aged 40 or over. (People aged 35-40 are accepted subject to being considered high risk, please inquire)
Currently have no symptoms of cancer. If you have suspicious symptoms, you should consult your GP urgently.
Do not currently have any clinical or radiological suspicion of cancer.
Have never been diagnosed with any cancer.
Have never received treatment for any type of cancer.
Do not receive a blood transfusion within 72 hours before your blood test appointment.
Sample tubes contain an additive or activator that must be mixed gently with the sample, to ensure sample stability.
The sample will remain stable during transport. It must be returned to the laboratory on the same day of collection. We have taken every precaution and tested different shipping conditions and temperature variations to ensure that the samples remain intact during transport to the laboratory.
In the rare event that there are transport delays or extreme temperatures and your sample cannot be tested by the laboratory, you will be contacted and our customer team will arrange for a new test kit to be sent out.
Included in the price is the test kit, home delivery, the DHL return envelope, the test report and an interview with a specialized doctor in the event of positive results who will be able to guide you towards the best protocol.
Not included in the test: The blood test to be carried out in the laboratory of your choice.
As this early cancer detection test is non-invasive, there is no downside to frequent testing. However, we advise carrying out this test every 12 months in the case of each negative result, perhaps as part of an annual comprehensive health check process.
-TruCheck detects the existence of cancers and not the possibility of cancers
- Conventional means of detecting cancer (mammography, colonoscopy, etc.) should still be favored
- A negative response to this test does not mean that you are free from any possibility of getting cancer
- TruCheck is not a substitute for medical diagnosis or treatment
The security of your data is of the utmost importance to us. We use appropriate technical and organizational measures to ensure the security and confidentiality of your information, and only share your information when necessary to provide our products and services or when we have a legal basis to do so. All samples are discarded after analysis.
For more information about our privacy and security practices, please review our Privacy Policy and Terms and Conditions or feel free to contact our Data Protection Officer at t.derville@Bodycheckup.com


The test can identify a wide range of solid organ cancers (cancers that affect the organs rather than the blood), including:
And much more